Medical Uses and Side Effects:
– Eszopiclone is used to treat insomnia with slight to moderate benefits for up to six months.
– Common side effects include headache, dry mouth, nausea, and dizziness.
– Severe side effects may include suicidal thoughts, hallucinations, and angioedema.
– Rapidly decreasing the dose may lead to withdrawal symptoms.
Elderly and Adverse Effects:
– Sedative hypnotic drugs like eszopiclone are more prescribed to the elderly despite limited benefits.
– The American Geriatrics Society recommends avoiding eszopiclone due to minimal efficacy and potential harms.
– Adverse effects include an increased risk of death, hypersensitivity, and liver impairment.
– Caution is advised for patients with certain medical conditions or activities requiring alertness.
Dependence and Non-Medical Use:
– Eszopiclone is a schedule IV controlled substance due to the risk of dependence.
– Tolerance may develop with prolonged use, especially in patients with a history of substance use disorders.
– Studies indicate potential for non-medical use and overdose risks with higher doses.
– Caution is advised due to the potential for misuse and adverse effects.
Pharmacology and History:
– Eszopiclone acts on benzodiazepine binding sites on GABA neurons.
– It has a rapid absorption after oral administration and is extensively metabolized.
– Studies on its effectiveness in treating insomnia have been controversial.
– The history includes findings from trials, efficacy data, and availability issues in Europe.
Dosage, Administration, Interactions, and Warnings:
– The recommended starting dose for adults is 1 mg, taken just before bedtime.
– Eszopiclone may interact with CNS depressants and should not be combined with alcohol.
– Precautions include avoiding use in individuals with a history of drug abuse, monitoring for mood changes, and caution in the elderly.
– Abrupt discontinuation may lead to rebound insomnia, and patients should inform healthcare providers of all medications being taken.
Eszopiclone, sold under the brand name Lunesta among others, is a medication used in the treatment of insomnia. Evidence supports slight to moderate benefit up to six months. It is taken by mouth.
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Lunesta, Eszop, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605009 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 52–59% |
| Metabolism | Liver oxidation and demethylation (CYP3A4 and CYP2E1-mediated) |
| Elimination half-life | 6 hours |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.149.304 |
| Chemical and physical data | |
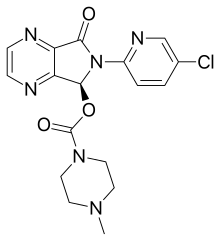
| Formula | C17H17ClN6O3 |
| Molar mass | 388.81 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Common side effects include headache, dry mouth, nausea, and dizziness. Severe side effects may include suicidal thoughts, hallucinations, and angioedema. Rapid decreasing of the dose may result in withdrawal. Eszopiclone is classified as a nonbenzodiazepine or Z-drug and a sedative and hypnotic of the cyclopyrrolone group. It is the S-stereoisomer of zopiclone. It works by interacting with the GABA receptors.
Approved for medical use in the United States in 2004, eszopiclone is available as a generic medication. In 2020, it was the 232nd most commonly prescribed medication in the United States, with more than 1 million prescriptions. Eszopiclone is not sold in the European Union, as of 2009, the European Medicines Agency (EMA) ruled that it was too similar to zopiclone to be considered a new active substance.
